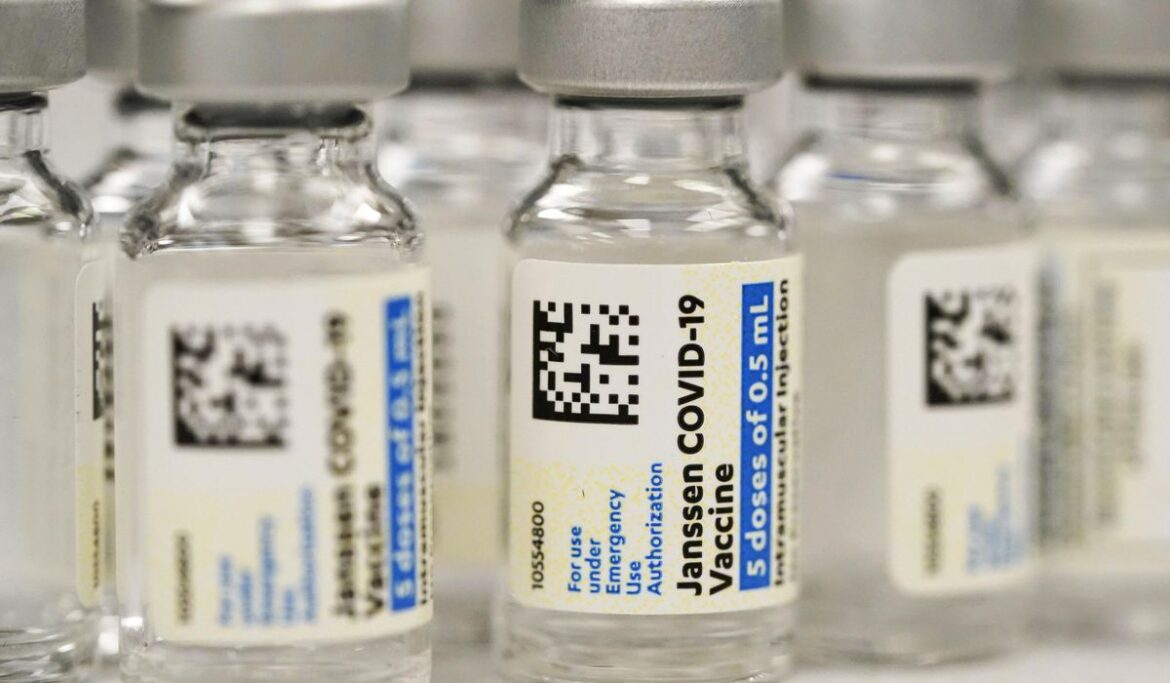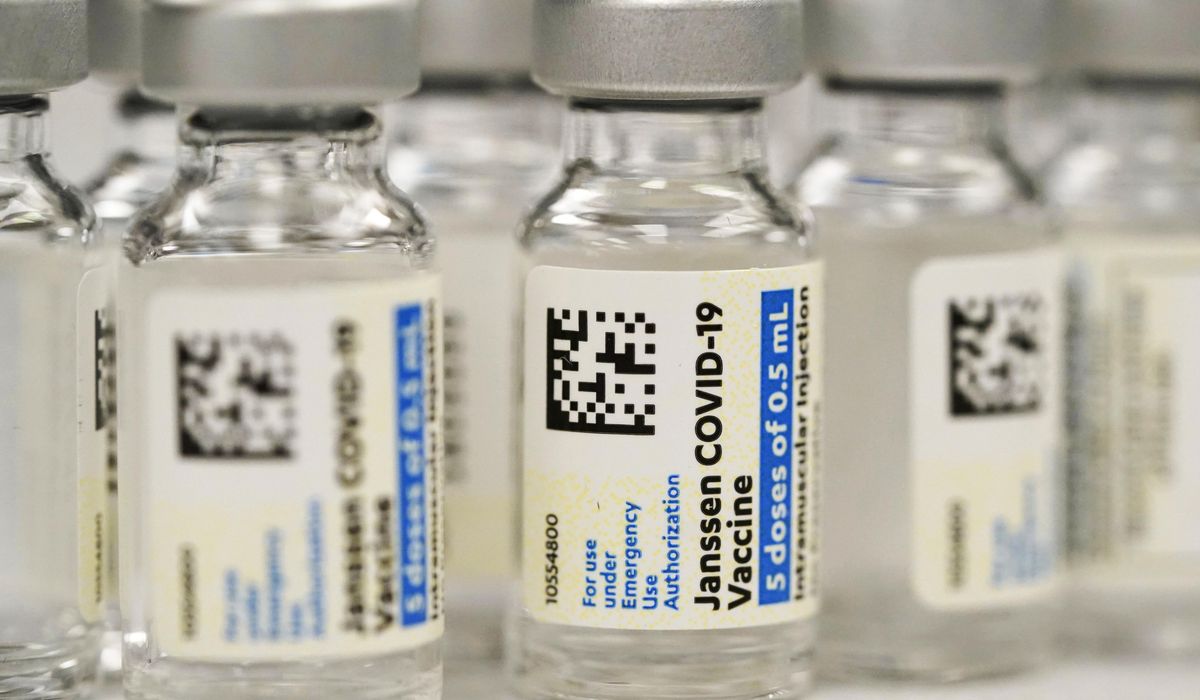
The Food and Drug Administration said Thursday it is limiting the use of the COVID-19 vaccine from Johnson & Johnson to adults who cannot access another version or might face a medical issue if they take another type.
Regulators cited the risk of a rare but severe blood-clotting condition that’s been linked to the J&J vaccine, with 60 known cases and nine deaths.
It is another setback for the J&J vaccine, which showed promise as an efficient one-shot option earlier in the pandemic, but faced manufacturing hiccups and questions about safety and efficacy versus the messenger RNA vaccines from Pfizer-BioNTech and Moderna that have become the dominant options.
“We recognize that the Janssen COVID-19 Vaccine still has a role in the current pandemic response in the United States and across the global community. Our action reflects our updated analysis of the risk of [thrombosis with thrombocytopenia syndrome] following administration of this vaccine and limits the use of the vaccine to certain individuals,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
Fewer than 20 million of the J&J shots have been administered in the U.S., and it accounts for less than 4% of primary vaccinations in the U.S. It also accounts for a small fraction of booster shots.
The vaccine has been part of the trinity of vaccines used in the U.S. since early 2021, but it was paused for a time in early 2021 as regulators investigated the rare clotting issue, or TTS.
It also faced manufacturing problems due to a mix-up at a Baltimore plant, and regulators previously said the mRNA vaccines were preferable to the J&J vaccine because of lingering concerns about TTS.
The FDA on Thursday said it took even stricter action because it feared rates of TTS were not trailing off.
“The FDA has determined that the known and potential benefits of the vaccine for the prevention of COVID-19 outweigh the known and potential risks for individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine,” the agency said.
The Washington Times reached out to J&J for comment on the FDA’s decision.
For more information, visit The Washington Times COVID-19 resource page.
RSS